Estimated reading time: 6.5 minutes
Authors: Jason Zhu (CHAI), Priscilla Rouyer (CHAI), Johanna Fihman (WHO), and Miloud Kaddar (LNCT)
In our previous blog, we discussed how Gavi transition and the resulting loss of access to Gavi-prices, increased financial burden, and loss of partner support may reduce a country’s ability to effectively procure vaccines. In this blog, we will go into greater detail on how countries can use market intelligence resources, many of which are publicly available, to help address these Gavi transition risks and ensure continued access to affordable and programmatically suitable vaccines. We will discuss how countries can find and use market intelligence information regarding product choice and price.
Product choice: which products are available in the market?
To introduce a new vaccine or optimize a country’s vaccine product portfolio, one first should understand the breadth of vaccine products currently available or soon-to-be available. In today’s market, this is increasingly important as more and more manufacturers develop innovative new products – many of which may be more affordable or programmatically suitable. Staying up-to-date on market developments could translate into significant procurement savings and programmatic benefits.
Resources
Below is a list of resources for countries to browse product options – focusing mostly on World Health Organization (WHO) pre-qualified (PQ) vaccines (Table 1). Gavi transitioned countries may find that publicly available resources developed for Gavi-eligible countries continue to be a useful reference resource even after transition (e.g., Gavi Detailed Product Profiles). Please note that countries participating in pooled procurement structures like the Pan-American Health Organization Revolving Fund (PAHO RF) may only have access to a specific set of products and presentations, as indicated in their pooled procurement menus.
Table 1. Vaccines Databases for Existing Products
| Vaccine ‘Group’ | Database | Information |
| WHO Pre-Qualified Vaccines | WHO’s Pre-Qualification Vaccine Database | Provides a brief profile of products’ key attributes like (but not limited to) manufacturer, responsible national regulatory agency (NRA), administration, presentation, shelf-life, vaccine vial monitor (VVM), packaging details, cold-chain volume. Link |
| Gavi Eligible Vaccines | Gavi Detailed Product Profiles | Includes information on key product attributes in WHO’s PQ database, plus additional information on product availability, price, and wastage for Gavi countries. Information on pipeline vaccines ~1 year from the market is sometimes available for PCV, Rota, and HPV; please refer to the product specific sheets. Link |
| UNICEF Procured Vaccines | UNICEF Vaccine Price Data | Historic, current, and future awarded prices for vaccines. Link |
| PAHO Procured Vaccines | PAHO Revolving Fund Vaccine Prices | The menu and price of PAHO supported vaccines. Link |
Pipeline Products
It is also important to understand which vaccines are projected to come into the market soon. Gavi’s detailed product profiles and antigen-specific product profiles, available for Pneumococcal Conjugate Vaccine (PCV), Rotavirus vaccine (Rota), and Human Papilloma Virus (HPV) vaccines, contain information about pipeline products that will likely be pre-qualified within a year (Table 2). More detailed information about the timing of availability and product attributes can be obtained by contacting local WHO and/or UNICEF program offices.
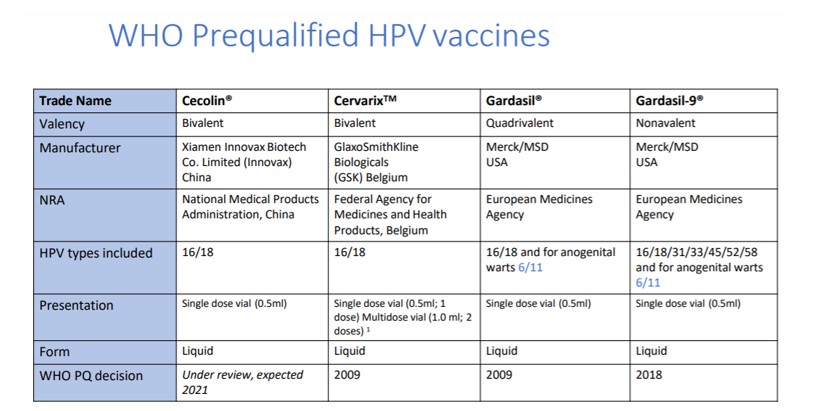
Table 2: As of February 2021, Human Papilloma Virus vaccine profiles slide-kit, including pipeline products and their estimated date of availability [1]
Product price: what is an ‘appropriate’ price for vaccines?
Long-term financial sustainability is one of the greatest challenges brought on by Gavi-transition. Losing access to Gavi-eligible prices will likely increase the cost of procurement over time but knowing how to use market intelligence can help limit the financial impact of transition by informing a variety of decisions like identifying savings opportunities, cost-effectiveness, product selection, price negotiations, and financial forecasting.
Given the variance in vaccine prices across products and countries, it is important to understand how to analyze prices and the various determinants of pricing. We recommend reviewing “Why understanding your country context is essential prior to using market intelligence insights”, in the first blog of our series.
There are multiple ways to evaluate the price of a vaccine and will depend on data availability (e.g., wastage), technical capacity, and the desired use-case. For example:
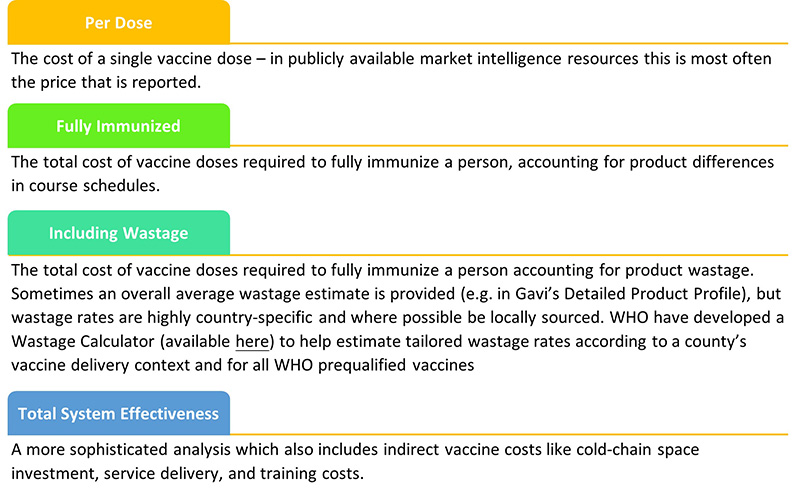
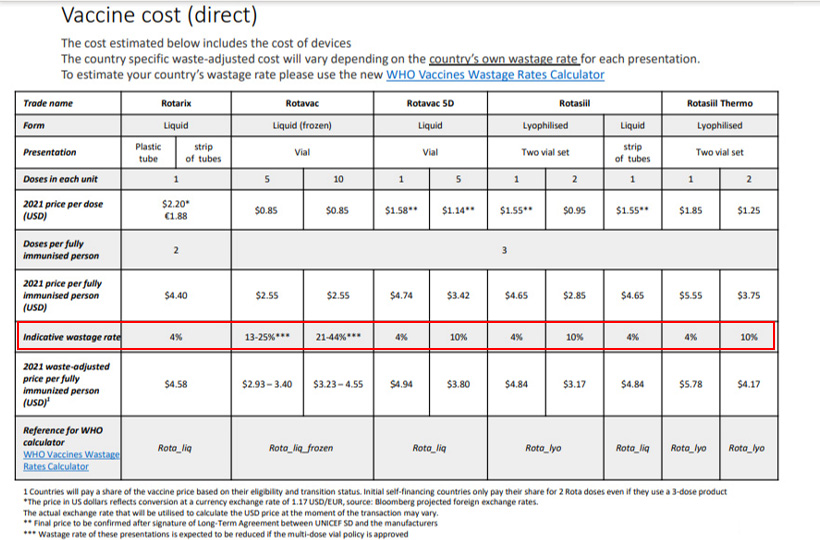
Here is an example on how to compare different Rota vaccines according to price per dose, per fully immunized child, and considering the wastage rate[2].
Several initiatives by global health partners have arisen in recent years to increase vaccine price transparency:
Pooled Procurement Menus
Countries procuring through a UNICEF, PAHO, or other pooled procurement model should look at the resources highlighted in Table 1 to understand pricing across eligible products. The prices listed in the PAHO vaccines menu may be of particular interest as they provide a reference floor price. This is because PAHO has negotiated with suppliers that for a particular product-presentation (e.g. Pfizer PCV13 1-dose presentation), PAHO must be able to access the lowest price offered in the world (excluding Gavi).
WHO MI4A Vaccine Price Database
The MI4A database contains country-anonymized vaccine price and procurement data for over 150 WHO member states and is structured to provide insight into how countries (grouped by GNI, procurement method, contract volume, etc.) pay for certain vaccines. WHO has already analyzed the available data and distilled these insights in a publicly available Global Vaccine Market Reports here. Countries can use this data to understand how vaccine prices may increase as they transition from Gavi support or other pricing trends in the vaccine market.
Vaccine manufacturer price commitments
The prices that Gavi eligible countries pay for co-financed vaccines often represent special access prices that non-Gavi countries are not eligible for. To help lessen the financial impact of Gavi transition vaccine manufacturer price commitments for HPV, PCV, and Rota vaccines were created to allow countries to pay Gavi-like prices after transition. Eligibility to access these Gavi-like prices varies by product and manufacturer. The specific criteria that determine eligibility are outlined in the following Gavi document. It is recommended that countries reach out to both Gavi and manufacturers to confirm eligibility to access these price-commitments.
Pneumococcal Conjugate and Rotavirus Vaccine Cost Calculators
PATH have developed a simple excel tool for assessing and comparing costs of PCV and Rotavirus vaccination programs with each PCV and Rota product available in the global market. These tools aim to help country-level policy makers compare products and estimate vaccination program costs for different products, exploring up to six difference vaccine options at a time. The tool can be used by any country interested in comparing difference vaccine costs. A HPV vaccine cost calculator is currently under development and should soon be available as well. These tools can be found here.
Information Sharing with Neighboring Countries
Where possible, countries should coordinate with neighboring or similar countries to share pricing information. Improved market intelligence coordination and sharing is the first step to setting up more sophisticated pooled procurement systems. Online platforms like the Vaccines Procurement Practitioners Network (VPPN) and the Learning Network for Countries in Transition (LNCT) offer opportunities to connect with procurement and vaccine program officers in other countries. Additionally, many regional vaccine bodies (e.g. RITAGs or regional working groups) are also working to improve vaccine coordination and collaboration across countries, including but not limited to procurement. In the next blog, we will discuss a case study on the benefits of cross-country market intelligence sharing and how it benefited the Republic of Congo and Cote d’Ivoire.
Up-next
As you can see countries can use market intelligence resources, many of which are publicly available, to ensure continued access to affordable and programmatically suitable vaccines. Knowing which vaccines are available and how much they cost is critical. Long-term financial sustainability is one of the greatest challenges brought on by Gavi-transition. And more and more manufacturers are developing innovative new products – many of which may be more affordable or programmatically suitable for your country. In the next and final series of the blog we will discuss where one can find information on supply shortages. And now that we know just how much information is publicly available, how one can develop strategies to keep up to date easily and effectively on vaccine markets.
We would like to acknowledge the contributions of the Bill and Melinda Gates Foundation, Gavi the Vaccine Alliance, UNICEF, and the Learning Network for Countries in Transition in reviewing, editing, and supporting the development of this blog series.
[1] https://www.gavi.org/sites/default/files/document/rotavirus-vaccine-profilespdf.pdf
[2] https://www.gavi.org/sites/default/files/document/guidelines/Gavi-Rotavirus-vaccines-profiles-ENG-102020.pdf
